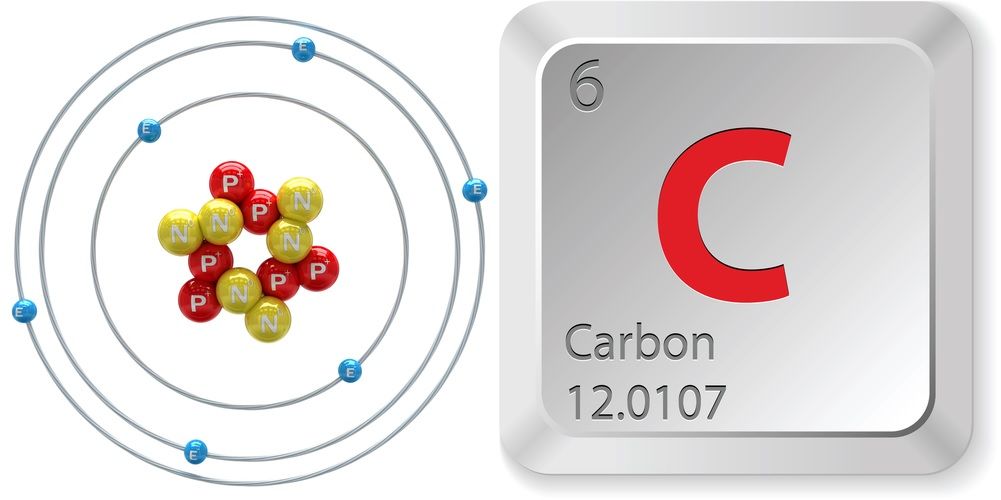
C Atom
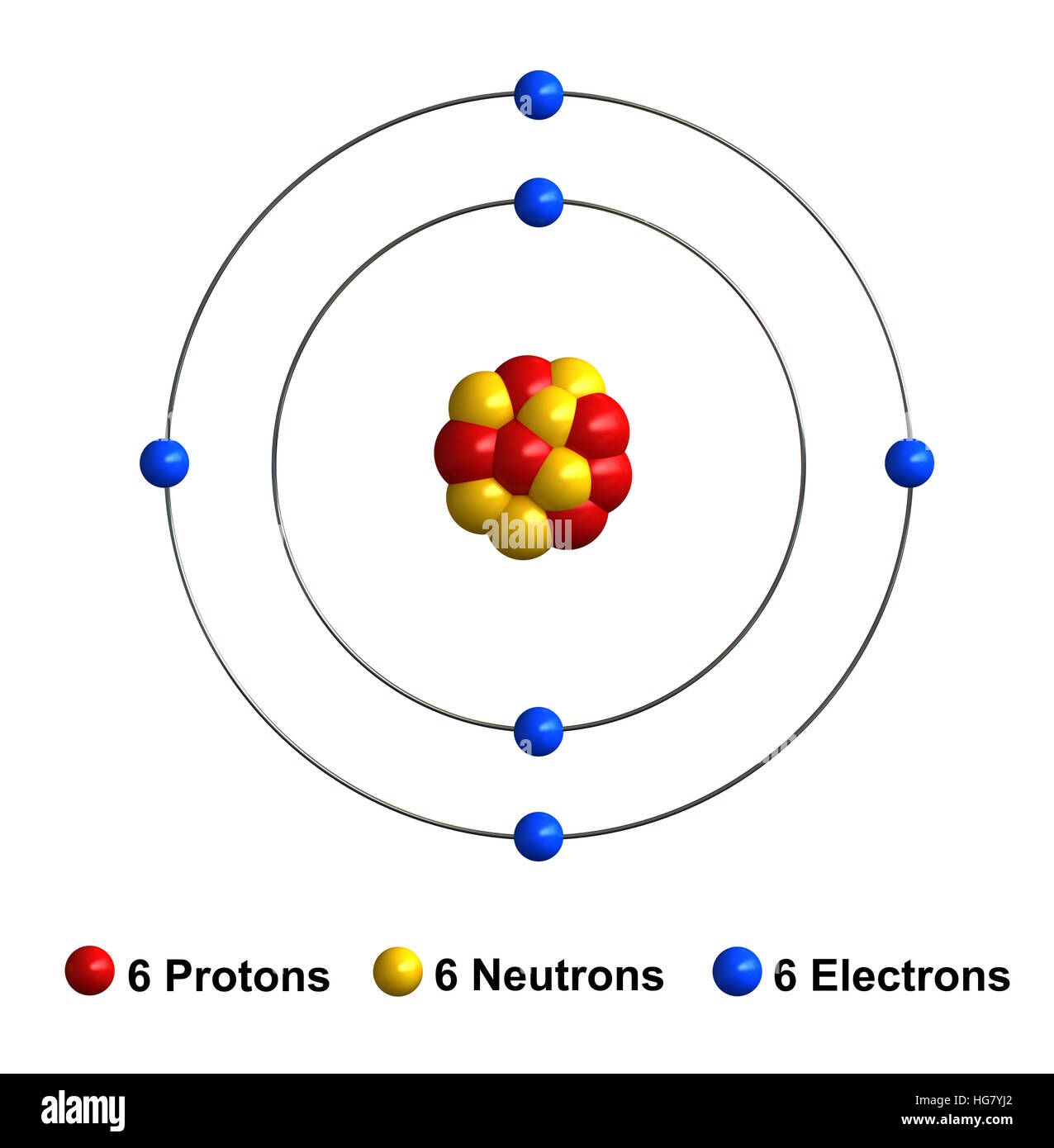
The carbon atom, in particular an atom of the isotope14C, has been chosen to illustrate the implications of quantum mechanics for the energy required to confine a particle to a region of space. Without recourse to the details of the nature of the fundamental forces, quantum mechanics gives the insight that it takes 10's of electron volts to contain electrons in atoms, and energies on the order of MeVs to contain protons in nuclei.
Taking the diameter of a carbon atom from the periodic table and calculating the minimum energy consistent with the uncertainty principle for a cubical volume of that dimension, we obtain a value of 10.4 eV. This compares with the observed value of 11.3 eV for the first ionization potential for the carbon atom. If we calculate the minimum confinement energy for the proton in a space the size of a carbon atom, we find that to be only 0.0056 eV. This extremely low energy can be compared with the average thermal energy of 0.04 eV at 300K! This implies that the proton, with only the energy provided by the internal energy of the normal environment can just wander in and out of an atomic space.
Trace the path of a carbon atom from Grandma Johnson's remains to where it could become part of a coyote. In your response include a drawing with arrows showing the movement of the C atom through various reservoirs (reservoir = pool or compartment with C, not necesarily a.
Carbon atoms are the building blocks for carbon, which is the sixth most abundant element found in the universe. A carbon atom has six electrons, four of which are in the outer shell of the atom, its valence shell. Carbon atoms have six protons in the nucleus and six electrons orbiting around the nucleus. Intel Atom® C processor, a system-on-a-chip (SoC) design, delivers efficient intelligence into smaller spaces at the network edge. Used in a variety of light scale-out workloads that require very low power, high density, and high I/O integration including network routers, switches, storage, security appliances, dynamic web serving, and more. The non-metallic element on which all organic chemistry is based and which is thus present in all organic matter. A carbon atom is capable of combining with up to four other atoms (tetravalent), including other carbon atoms; it is this property that allows so many compounds to be formed.
When we apply the minimum energy calculation to the carbon nucleus, the picture is very different. The confinement energy for keeping a proton inside the a cubical volume of dimension equal to the nuclear diameter is 5.6 MeV. This compares well in magnitude with the observed average binding energy of 7.5 MeV for nucleons in the carbon-14 nucleus. So with no tools other than the uncertainty principle, we have established the scale of energy for nuclear processes. Observed radioactive processes are in the range 0-10 MeV, and this is consistent with the uncertainty principle.
The story for the electron is more dramatic. We observe electrons being emitted from the carbon-14 nucleus (beta decay) with the relatively small energy of 0.016 MeV. Does that imply that there are electrons hanging around inside the carbon nucleus? Definitely not!! The minimum confinement energy for an electron in the nuclear volume is a preposterously high 10.2 GeV, a half-million times greater than the observed decay energy of the carbon-14 nucleus. The calculation of required confinement energy then implies that the observed electron has been created inside the nucleus as a part of the radioactive decay process rather than being simply an ejection of an electron which was already there.
What is the hybridization in #'CO'_2#?
1 Answer
The carbon atom has
Explanation:
You must first draw the Lewis structure for
According to VSEPR theory, we can use the steric number
#'SN = 2'# corresponds to#sp# hybridization.#'SN'= 3'# corresponds to#sp^2# hybridization.
We see that the
It has
Each
Just as the carbon atom hybridized to form the best bonds, so do the oxygen atoms.
The valence electron configuration of
6.022x10 23 Atoms C
To accommodate the two lone pairs and the bonding pair, it will also form three equivalent
Two of the
We can see this arrangement in the
(from www.slideshare.net)
C Atom Number
There is a similar arrangement on the left side of the
Here is a video about the hybridization of carbon dioxide.
C Atomic Symbol
Related questions
