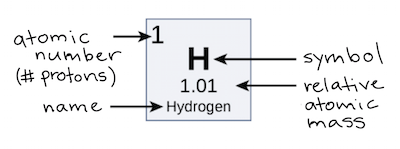
The Atomic Mass Of An Element Is
The mass written on the periodic table is an average atomic mass taken from all known isotopes of an element. This average is a weighted average, meaning the isotope's relative abundance changes its impact on the final average. The reason this is done is because there is no set mass for an element. The measurement unit used for atomic mass is the unified atomic mass that has the symbol 'u.' This is a derived unit from the carbon-12 isotope, where 12 u is the atomic mass of carbon-12. On the periodic table, students can find the atomic mass written below the element's name.
Taken from http://www.sizes.com/units/atomic_mass_unit.htm
History of the atomic mass unit

Stanislao Cannizzaro (1826–1910), the pioneer in this field, adopted the hydrogen atom as a standard of mass and set its atomic weight at 2. Others accepted the idea of using a specific atom as a standard of mass, but preferred a more massive standard in order to reduce experimental error.
As early as 1850, chemists used a unit of atomic weight based on saying the atomic weight of oxygen was 16. Oxygen was chosen because it forms chemical compounds with many other elements, simplifying determination of their atomic weights. Sixteen was chosen because it was the lowest whole number that could be assigned to oxygen and still have an atomic weight for hydrogen that was not less than 1.
The 0=16 scale was formalized when a committee appointed by the Deutsche Chemische Gesellschaft called for the formation of an international commission on atomic weights in March 1899. A commission of 57 members was formed. Since the commission carried on its business by correspondence, the size proved unwieldy, and the Gesellschaft suggested a smaller committee be elected. A 3-member International Committee of Atomic Weights was duly elected, and in 1903 issued its first report, using the 0=16 scale.5
Taking isotopes into account
The discovery of isotopes complicated the picture. In nature, pure oxygen is composed of a mixture of isotopes: some oxygen atoms are more massive than others.
This was no problem for the chemists’ calculations as long as the relative abundance of the isotopes in their reagents remained constant, though it confirmed that oxygen’s atomic weight was the only one that in principle would be a whole number (hydrogen’s, for example, was 1.000 8).
Physicists, however, dealing with atoms and not reagents, required a unit that distinguished between isotopes. At least as early as 19276 physicists were using an atomic mass unit defined as equal to one-sixteenth of the mass of the oxygen-16 atom (the isotope of oxygen containing a total of 16 protons and neutrons).
In 1919, isotopes of oxygen with mass 17 and 18 were discovered.7 Thus the two amu’s clearly diverged: one based on one-sixteenth of the average mass of the oxygen atoms in the chemist’s laboratory, and the other based on one-sixteenth of the mass of an atom of a particular isotope of oxygen.
In 1956, Alfred Nier (at the bar in the Hotel Krasnapolski in Amsterdam) and independently A. Ölander8, both members of the Commission on Atomic Masses of the IUPAP, suggested to Josef Mattauch that the atomic weight scale be based on carbon-12. That would be okay with physicists, since carbon-12 was already used as a standard in mass spectroscopy. The chemists resisted making the amu one-sixteenth the mass of an oxygen-16 atom; it would change their atomic weights by about 275 parts per million. Making the amu one-twelfth the mass of a carbon-12 nucleus, however, would lead to only a 42 parts per million change, which seemed within reason.
Mattauch set to work enthusiastically proselytizing the physicists, while E. Wichers lobbied the chemists.9 In the years 1959–1961 the chemists and physicists resolved to use the isotope carbon-12 as the standard, setting its atomic mass at 12.
How do I calculate the average atomic mass of element X?
isotope: abundance: mass:
221x 74.22 220.9
220x 12.78 220.0
218x 13.00 218.1
isotope: abundance: mass:
221x 74.22 220.9
220x 12.78 220.0
218x 13.00 218.1
1 Answer

Here's how I do it.
The atomic mass is the weighted average of the atomic masses of each isotope.
In a weighted average, we multiply each value by a number representing its relative importance.

In this problem, the percent abundance represents the relative importance of each isotope.
Here's how to do the calculation:
The Atomic Mass Of An Element Is Calculated Using The
The average relative atomic mass of element X is 220.4.
The Atomic Mass Of An Element Is Quizlet
Related questions